Growing organicNature 412, 517–520 (2 August 2001) Imagine how useful a plastic transistor could be. Flexible displays and cheap, all-plastic smart cards are just a few possibilities. Last year, the Nobel Prize for chemistry went to Heeger, MacDiarmid and Shirakawa for their discovery of electrically conducting polymers, the committee predicting "consequences of great benefit to mankind".
But still most microelectronic devices are made of inorganic
semiconducting materials. Plastic transistors are already showing
promise for applications , but their speed of operation is
slow compared to their inorganic counterparts, limiting their
utility. The problem stems from the difficulty in making organic
crystals of high quality in thin-film form. But that may be
about to change: this week in Nature, Frank Meyer zu
Heringdorf and colleagues report progress in understanding organic
thin-film growth through detailed studies of the semiconducting
molecule pentacene (C22H14), a chain-like
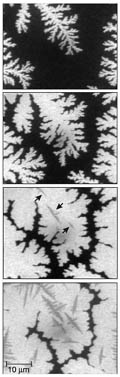
aromatic molecule made of five benzene rings. Using photoelectron emission microscopy, with a field of view of up to 65 µm and a resolution of 125 nm, the authors watched the build-up of pentacene on a Si(001) surface over a timescale of minutes. First, stable two-dimensional islands of pentacene nucleate on the surface, each island forming a one-layer-thick pentacene crystal. The islands branch out like fractals, but before they touch, a second layer nucleates (typically when the first layer coverage reaches about 60%). Eventually, the trenches between islands are filled in, leaving grain boundaries that act as barriers to charge flow — and which would hinder the electrical performance of transistors made from such films. Meyer zu Heringdorf et al. noticed a dead-time between the start of deposition and the nucleation of pentacene islands. Further imaging revealed that the first-deposited pentacene molecules are adsorbed at reactive sites (where there are 'dangling bonds') on the Si(001) surface. Only when all these sites are neutralized by the adsorption of pentacene can the first layer of the thin crystalline film start to grow. Treating the Si surface with cyclohexene or using a different substrate such as SiO2, which has no dangling bonds, got rid of the dead-time and produced smoother films. Also reported in the paper is the successful growth of films
with grain sizes reaching about 0.1 mm — about 100 times larger
than in previous work, and opening up the possibility of fabricating
an organic transistor entirely contained within a single grain.
The authors conclude that their observations will speed up the
development of workable organic thin-film devices. letters to nature |