Among the promised fruits of nanotechnology, small machines have always
stood out. Their attraction is straightforward. Large machines--airplanes,
submarines, robotic welders, toaster ovens--are unquestionably useful. If
one could take the same ideas used to design these devices and apply them
to machines that were a tiny fraction of their size, who knows what they
might be able to do? Imagining two types of small machines--one analogous
to an existing machine, the second entirely new--has captured broad
attention. The first is a nanoscale submarine, with dimensions of only a
few billionths of a meter--the length of a few tens or hundreds of atoms.
This machine might, so the argument goes, be useful in medicine by
navigating through the blood, seeking out diseased cells and destroying
them.
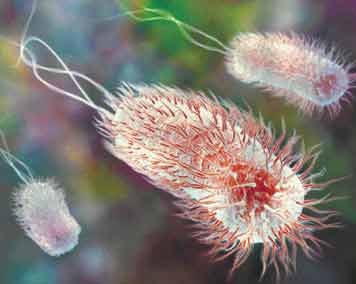 |
|
Image Credit--JEFF JOHNSON Hybrid Medical
Animation
WHIPLIKE TAILS, found on many
bacteria, are propelled by nanomotors. The tiny biochemical motor
turns a rotary shaft that spins the tails, or flagella, and allows
the bacteria, such as these E. coli, to move through
liquid. |
The second--the so-called
assembler--is a more radical idea, originally proposed by futurist K. Eric
Drexler. This machine has no macroscopic analogue (a fact that is
important in considering its ultimate practicality). It would be a new
type of machine--a universal fabricator. It would make any structure,
including itself, by atomic-scale "pick and place": a set of nanoscale
pincers would pick individual atoms from their environment and place them
where they should go. The Drexlerian vision imagines society transformed
forever by small machines that could create a television set or a computer
in a few hours at essentially no cost. It also has a dark side. The
potential for self-replication of the assembler has raised the prospect of
what has come to be called gray goo: myriads of self-replicating
nanoassemblers making uncountable copies of themselves and ravaging the
earth while doing so.
Does the idea of nanoscale machines make sense? Could they be made? If
so, would they be effectively downsized versions of their familiar,
large-scale cousins, or would they operate by different principles? Might
they, in fact, ravage the earth?
We can begin to answer these intriguing questions by asking a more
ordinary one: What is a machine? Of the many definitions, I choose to take
a machine to be "a device for performing a task." Going further, a machine
has a design; it is constructed following some process; it uses power; it
operates according to information built into it when it is fabricated.
Although machines are commonly considered to be the products of human
design and intention, why shouldn't a complex molecular system that
performs a function also be considered a machine, even if it is the
product of evolution rather than of design?
|
| The charm of the assembler is illusory: it is more appealing as
metaphor than as reality, and less the solution of a problem than
the hope for a miracle. |
|
Issues of teleology aside, and accepting this
broad definition, nanoscale machines already do exist, in the form of the
functional molecular components of living cells--such as molecules of
protein or RNA, aggregates of molecules, and organelles ("little
organs")--in enormous variety and sophistication. The broad question of
whether nanoscale machines exist is thus one that was answered in the
affirmative by biologists many years ago. The question now is: What are
the most interesting designs to use for future nanomachines? And what, if
any, risks would they pose?
Cells include some molecular machines that seem similar to familiar
human-scale machines: a rotary motor fixed in the membrane of a bacterium
turns a shaft and superficially resembles an electric motor. Others more
loosely resemble the familiar: an assembly of RNA and protein--the
ribosome--makes proteins by an assembly line–like process. And some
molecular machines have no obvious analogy in macroscopic machines: a
protein--topoisomerase--unwinds double-stranded DNA when it becomes too
tightly wound. The way in which these organelles are fabricated in the
cell--an efficient synthesis of long molecules, combined with molecular
self-assembly--is a model for economy and organization, and entirely
unlike the brute-force method suggested for the assembler.
And as for ravaging the earth: in a sense, collections of biological
cells already have ravaged the earth. Before life emerged, the planet was
very different from the way it is today. Its surface was made of inorganic
minerals; its atmosphere was rich in carbon dioxide. Life rapidly and
completely remodeled the planet: it contaminated the pristine surface with
microorganisms, plants and organic materials derived from them; it largely
removed the carbon dioxide from the atmosphere and injected enormous
quantities of oxygen. Overall, a radical change. Cells--self-replicating
collections of molecular nanomachines--completely transformed the surface
and the atmosphere of our planet. We do not normally think of this
transformation as "ravaging the planet," because we thrive in the present
conditions, but an outside observer might have thought otherwise.
So the issue is not whether nanoscale machines can exist--they already
do--or whether they can be important--we often consider ourselves as
demonstrations that they are--but rather where we should look for new
ideas for design. Should we be thinking about the General Motors assembly
line or the interior of a cell of E. coli? Let's begin by comparing
biological nanomachines--especially the ultimate self-replicating
biological system, the cell--with nanoscale machines modeled on the large
machines that now surround us. How does the biological strategy work, and
how would it compare with a strategy based on making nanoscale versions of
existing machines, or a new strategy of the type suggested by the
assembler?
Molecular Copy Machines
The cell is a self-replicating structure. It takes in molecules from
its environment, processes some of them for fuel, and reworks others into
the pieces it uses to make, maintain, move and defend itself. DNA stores
the information needed for fabrication and operation from one generation
to the next. One kind of RNA (messenger RNA, or mRNA) serves as the
temporary transcript of this information, "telling" ribosomes which
protein to make. Membranes provide compartments that enclose the working
parts, house portals that control the flux of molecules into and out of
the cell, and hold molecules that sense the cell's environment. Proteins
(often cooperating with other molecules) build everything in the cell and
move its parts when they must be moved.
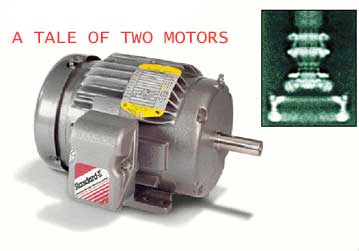 |
|
Image Credit--Baldor Electric Company;
DAVID DEROSIER, © 2000 American Institute of Physics
(micrograph)
STANDARD-ISSUE electric motor bears a
superficial--albeit striking--resemblance to the biochemical rotary
motor (top right) that turns the flagella in a
bacterium. |
The strategy adopted by the cell
to make its parts--and thus to replicate and maintain itself--is based on
two ideas. The first is to use a single, conceptually straightforward
chemical process--polymerization--to create large, linear molecules. The
second is to build molecules that spontaneously fold themselves into
functional, three-dimensional structures. This two-part strategy does not
require a difficult and sophisticated three-dimensional pick-and-place
fabrication: it simply strings beads (for example, amino acids) together
into a necklace (a polypeptide) and lets the necklace self-assemble into a
machine (a protein). Thus, the information for the final, functional,
three-dimensional structure is coded in the sequence of the beads. The
three most important classes of molecules in the cell--DNA, RNA and
proteins--are all made by this strategy; the proteins then make the other
molecules in the cell. In many instances proteins also spontaneously
associate with other molecules--proteins, nucleic acids, small
molecules--to form larger functional structures. As a strategy for
building complex, three-dimensional structures, this method of linear
synthesis followed by various levels of molecular self-assembly is
probably unbeatable for its efficiency.
The cell is, in essence, a collection of catalysts (molecules that
cause chemical reactions to occur without themselves being consumed) and
other functional species--sensors, structural elements, pumps, motors.
Most of the nanomachines in the cell are thus, ultimately, molecular
catalysts. These catalysts do most of the work of the cell: they form the
lipids (fats, for instance) that in turn self-assemble into the flexible
sheet that encloses the cell; they make the molecular components necessary
for self-replication; they produce the power for the cell and regulate its
power consumption; they build archival and working information storage;
and they maintain the interior environment within the proper operating
parameters.
Among the many marvelous molecular machines employed by the cell, four
are favorites of mine. The ribosome, made of ribosomal RNA (rRNA) and
protein, is a key: it stands at the junction between information and
action--between nucleic acids and proteins. It is an extraordinarily
sophisticated machine that takes the information present in mRNA and uses
it to build proteins.
|
| Considering the many constraints on the construction and
operation of nanomachines, it seems that new systems for building
them might ultimately look much like the ancient systems of
biology. |
|
The chloroplast, present in plant cells and
algae, is a large structure that contains arrays of molecules that act as
tuned optical antennas, collect photons from sunlight and employ them to
generate chemical fuel that can be stored in the cell to power its many
operations. The chloroplast, incidentally, also converts water to the
oxygen that so contaminated the atmosphere when life first emerged: the
stuff on which our lives depend was originally a waste product of cellular
light-harvesting!
The mitochondrion is the power station: it carries out controlled
combustion of organic molecules present in the cell--typically
glucose--and generates power for the system. Instead of pumping electrons
through wires to run electric motors, it generates molecules of ATP that
move through the cell by diffusion and that are essential contributors to
many biological reactions.
The flagellar motor of bacteria is a specialized but particularly
interesting nanomachine, because it seems so similar to human-scale
motors. The flagellar motor is a highly structured aggregate of proteins
anchored in the membrane of many bacterial cells that provides the rotary
motion that turns the flagella--the long whiplike structures that act as
the propeller for these cells and allow them to propel themselves through
water. It has a shaft, like an electric motor, and a structure around the
shaft, like the armature of a motor. The similarity between flagellar and
electrical motors is, however, largely illusory. The flagellar motor does
not act by using electric current to generate moving magnetic fields;
instead it uses the decomposition of ATP to cause changes in the shape of
the molecules that, when combined with a sophisticated molecular ratchet,
make the protein shaft revolve.
Nanomachines That Mimic Human-Scale Machines
Can we ever approach the elegant efficiency of cellular nanomachines by
creating tiny cousins of the larger machines we have invented?
Microfabrication has developed as an extraordinarily successful technology
for manufacturing small, electronically functional devices--transistors
and the other components of chips. Application of these techniques to
simple types of machines with moving parts--mechanical oscillators and
movable mirrors--has been technically successful. The development of these
so-called microelectromechanical systems (MEMS) is proceeding rapidly, but
the functions of the machines are still elementary, and they are micro,
not nano, machines. The first true nanoscale MEMS (NEMS, or
nanoelectromechanical systems) have been built only in the past few years
and only experimentally [see "Plenty of Room, Indeed," on page 48].
Many interesting problems plague the fabrication of nanodevices with
moving parts. A crucial one is friction and sticking (sometimes combined
in talking about small devices in the term "stiction"). Because small
devices have very large ratios of surface to volume, surface effects--both
good and bad--become much more important for them than for large devices.
Some of these types of problems will eventually be resolved if it is
worthwhile to do so, but they provide difficult technical challenges now.
We will undoubtedly progress toward more complex micromachines and
nanomachines modeled on human-scale machines, but we have a long path to
travel before we can produce nanomechanical devices in quantity for any
practical purpose. Nor is there any reason to assume that nanomachines
must resemble human-scale machines.
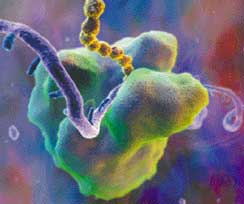 |
|
JEFF JOHNSON Hybrid Medical
Animation
RIBOSOME reads along a strand of RNA
(purple) to get instructions for stringing together the amino
acids that constitute a protein (gold). This assembly-line
process brings to mind the robotic welders in an automotive factory
(below). |
 |
|
MICHAEL S. YAMASHITA
Corbis |
Could these systems
self-replicate? At present, we do not know how to build self-replicating
machines of any size or type. We know, from recent biological studies,
something about the minimum level of complexity in a living cell that will
sustain self-replication: a system of some 300 genes is sufficient for
self-replication. We have little sense for how to translate this number
into mechanical machines of the types more familiar to us, and no sense of
how to design a self-sustaining, self-replicating system of machines. We
have barely taken the first steps toward self-replication in nonbiological
systems [see "Go Forth and Replicate," by Moshe Sipper and James A.
Reggia; Scientific American, August].
And other problems cast long shadows. Where is the power to come from
for an autonomous nanomachine? There are no electric sockets at the
nanoscale. The cell uses chemical reactions of specific compounds to
enable it to go about its business; a corresponding strategy for nanoscale
machines remains to be developed. How would a self-replicating nanomachine
store and use information? Biology has demonstrated a strategy based on
DNA, so it can be done, but if one wanted a different strategy, it is not
clear where to start.
The assembler, with its pick-and-place pincers, eliminates the many
difficulties of fabricating nanomachines and of self-replication by
ignoring them: positing a machine that can make any composition and any
structure by simply placing atoms one at a time dismisses the most vexing
aspects of fabrication. The assembler seems, however, from the vantage of
a chemist, to be unworkable. Consider just two of the constraints.
First is the pincers, or jaws, of the assembler. If they are to pick up
atoms with any dexterity, they should be smaller than the atoms. But the
jaws must be built of atoms and are thus larger than the atom they must
pick and place. (Imagine trying to build a fine watch with your fingers,
unaided by tools.) Second is the nature of atoms. Atoms, especially carbon
atoms, bond strongly to their neighbors. Substantial energy would be
needed to pull an atom from its place (a problem for the energy supply)
and substantial energy released when it is put in place (a problem of
cooling). More important, a carbon atom forms bonds with almost
everything. It is difficult to imagine how the jaws of the assembler would
be built so that, in pulling the atoms away from their starting material,
they would not stick. (Imagine trying to build your watch with parts
salvaged from another watch in which all the parts were coated with a
particularly sticky glue: if you could separate the pieces at all, they
would stick to your fingers.) Problems with the assembler are also
discussed by Richard E. Smalley in his essay on page 76.
Would a nanosubmarine work if it could be built? A human-scale
submarine moves easily in water by a combination of a rotating
propeller--which, in spinning, forces the water backward and the submarine
forward--and movable planes that guide its direction. Bacteria that swim
actually use structures--flagella--that look more like flexible spirals or
whips but serve a function similar to a propeller. They typically do not
steer a very purposeful path but rather dash about, with motion that, if
all goes well, tends in the general direction of a source of food. For
nanoscale objects, even if one could fabricate a propeller, a new and
serious problem would emerge: random battering by water molecules.
These water molecules would be smaller than a nanosubmarine but not
much smaller, and their thermal motion is rapid on the nanoscale.
Collisions with them make a nanoscale object bounce about rapidly (a
process called Brownian motion) but in random directions: any effort to
steer a purposeful course would be frustrated by the relentless collisions
with rapidly moving water molecules. Navigators on the nanoscale would
have to accommodate to the Brownian storms that would crash against their
hulls. For ships of approximately 100 nanometers in scale, the destination
of most voyages would be left to chance, because the tiny craft would
probably be impossible to steer, at least in a sense familiar to a
submariner. Cells in the bloodstream--objects 10 or 100 times more massive
than a nanosubmarine--do not guide themselves in it: they simply tumble
along with it. At best, a nanosubmarine might hope to select a general
direction but not a specific destination. Regardless of whether one could
make or steer devices at the nanoscale, they would not work for the
sophisticated tasks required to detect disease if one could make them.
Parts of the "little submarine" strategy for detecting and destroying
diseased cells in the body, such as cancer cells, would have to focus on
finding their prey. In doing so, they would probably have to mimic aspects
of the immune system now functioning in us. The recognition of a cell as
"normal" or "pathogen" or "cancer" is an extraordinarily complex
process--one that requires the full panoply of our immune system,
including the many billions of specialized cells that constitute it. No
simple markers on the outside of most cancer cells flag them as dangerous.
In many of their characteristics, they are not enormously different from
normal cells. A little submarine that was to be a hunter-killer for cancer
cells would have to carry on board a little diagnostic laboratory, and
because that laboratory would require sampling devices and reagents and
reaction chambers and analytical devices, it would cease to be little.
Operating this device would also require energy. The cells of the immune
system use the same nutrients as do other cells; a little submarine would
probably have to do the same.
Outdesigning Evolution
Small machines will eventually be made, but the strategy used to make
them, and the purposes they will serve, remain to be devised. Biology
provides one brilliantly developed set of examples: in living systems,
nanomachines do exist, and they do perform extraordinarily sophisticated
functions. What is striking is how different the strategies used in these
nanometer-scale machines are from those used in human-scale machines.
|
| It will be a marvelous challenge to see if we can outdesign
evolution. It would be a staggering accomplishment to mimic the
simplest living cell. |
|
In thinking about how best to make nanomachines,
we come up against two limiting strategies. The first is to take existing
nanomachines--those present in the cell--and learn from them. We will
undoubtedly be able to extract from these systems concepts and principles
that will enable us to make variants of them that will serve our purposes,
and others that will have entirely new functions. Genetic engineering is
already proceeding down this path, and the development of new types of
chemistry may enable us to use biological principles in molecular systems
that are not proteins and nucleic acids.
The second is to start from scratch and independently to develop
fundamental new types of nanosystems. Biology has produced one practical
means for fabrication and synthesis of functional nanomachines, and there
is no reason to believe that there cannot be others. But this path will be
arduous. Looking at the machines that surround us and expecting to be able
to build nanoscale versions of them using processes analogous to those
employed on a large scale will usually not be practical and in many cases
impossible. Machining and welding do not have counterparts at nanometer
sizes. Nor do processes such as moving in a straight line through a fluid
or generating magnetic fields with electromagnets. Techniques devised to
manufacture electronic devices will certainly be able to make some simple
types of mechanical nanodevices, but they will be limited in what they can
do.
The dream of the assembler holds seductive charm in that it appears to
circumvent these myriad difficulties. This charm is illusory: it is more
appealing as metaphor than as reality, and less the solution of a problem
than the hope for a miracle. Considering the many constraints on the
construction and operation of nanomachines, it seems that new systems for
building them might ultimately look much like the ancient systems of
biology. It will be a marvelous challenge to see if we can outdesign
evolution. It would be a staggering accomplishment to mimic the simplest
living cell.
Are biological nanomachines, then, the end of the line? Are they the
most highly optimized structures that can exist, and has evolution sorted
through all possibilities to arrive at the best one? We have no general
answer to this question. Jeremy R. Knowles of Harvard University has
established that one enzyme--triose phosphate isomerase, or TIM--is
"perfect": that is, no catalyst for the particular reaction catalyzed by
this enzyme could be better. For most enzymes, and all structures more
complicated than enzymes, we have made no effort to discover the
alternatives.
Biological structures work in water, and most work only in a narrow
range of temperatures and concentrations of salts. They do not, in
general, conduct electricity well (although some, such as the chloroplast
and the mitochondrion, move electrons around with great sophistication).
They do not carry out binary computation and communications. They are not
particularly robust mechanically. Thus, a great many types of function
must be invented if nanomachines are to succeed in nonbiological
environments.
And what have we learned from all this about the doomsday scenario of
gray goo? If a hazard were to arise from nanomachines, it would lie in a
capability for self-replication. To be self-replicating, a system must
contain all the information it needs to make itself and must be able to
collect from its environment all the materials necessary both for energy
and for fabrication. It must also be able to manufacture and assemble (or
allow to assemble) all the pieces needed to make a copy of itself. Biology
has solved all these problems, and self-replicating biological
systems--from pathogenic bacteria to cancer cells--are a danger to us. In
computer systems, self-replicating strings of bits (computer viruses),
although not material objects, are also at least a great nuisance, but
only indirectly a danger, to us.
If a new system--any system--were able to replicate itself using
materials present in the environment, it would be cause for concern. But
we now know enough to realize how far we are from reproducing
self-replication in a nonbiological system. Fabrication based on the
assembler is not, in my opinion, a workable strategy and thus not a
concern. For the foreseeable future, we have nothing to fear from gray
goo. If robust self-replicating micro (or perhaps nano) structures were
ultimately to emerge, they would probably be chemical systems as complex
as primitive bacteria. Any such system would be both an incredible
accomplishment and a cause for careful assessment. Any threat will not be
from assemblers gone amok but from currently unimaginable systems of
self-catalyzing reactions.
So biology and chemistry, not a mechanical engineering textbook, point
in the direction we should look for answers--and it is also where our
fears about organisms or devices that multiply uncontrollably are most
justified. In thinking about self-replication, and about the
characteristics of systems that make them "alive," one should start with
biology, which offers a cornucopia of designs and strategies that have
been successful at the highest levels of sophistication. In tackling a
difficult subject, it is sensible to start by studying at the feet of an
accomplished master. Even if they are flagella, not feet.