 |
 Using plants as plants Using plants as plants
|
| ROLF BACHMANN,
ENRICO BASTIANELLI, JENS RIESE, AND WIEBKE SCHLENZKA
|
| The McKinsey
Quarterly, 2000 Number 2, pp. 92–99 |
 |
Return to index Life Sciences Publications
Companies that make chemicals1 have been searching for new sources of
growth over the past decade. The quest has prompted some
chemical conglomerates to spin off their traditional chemical
businesses, such as commodity2 and specialty chemicals, to
concentrate on more profitable, noncyclical life science
enterprises. But the hopes these companies had of
realizing synergies among their life science
businesses—especially in biotechnology, which they all
use—have been largely unfulfilled.
This is not the
moment, however, for chemical companies to give up on
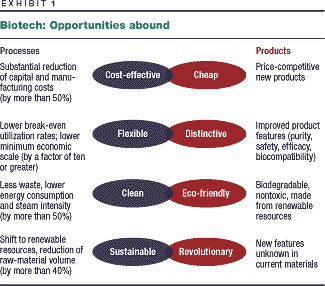
biotechnology. It still offers huge opportunities (Exhibit 1),
and two trends suggest that they are worth exploring. During
this century, science will continue to advance its
understanding of living organisms and how to control them. At
the same time, humanity's search for sustainable resources
will intensify. Using biotechnology to produce chemicals lies
squarely at the intersection of these trends. Although the
risks are substantial, they can be managed. Chemical companies
should therefore revisit their biotechnology strategies now.
RISK
MANAGEMENT
Biotechnology was born in the late
1970s following the development of techniques for recombining
genetic material. Today, pharmaceutical companies are the big
users of biotechnology, though they find it risky and
expensive. Traditional pharmaceutical companies put an average
of 15 percent of their annual revenues into research and
development; typical large biotech companies, more than 30
percent. The payback is slow. R&D for a single innovative
product costs up to $350 million—in biotechnology, there are
no sensible small-scale investments—and takes five to ten
years. Moreover, many products do not perform as hoped. And
even if a company does produce an effective biotech product,
consumers may reject it out of fear that it could damage the
environment, human health, or both.
Given these risks,
how should chemical companies go about investing in
biotechnology? By emulating the techniques venture capital
firms use to manage the risks of investing in pharmaceutical
biotechnology. Biotech venture capital funds do as well as
other types on average, and some of the first start-ups have
outperformed the blue chips.
USING
BIOTECHNOLOGY TO MAKE CHEMICALS
Today, the market for biotech-based basic and intermediate chemicals, fine chemicals,
polymers, and specialty chemicals accounts for only 2 percent
of the total chemical market, or about $25 billion in revenue.
3 But biotechnology, thanks to its cost,
scale, and quality advantages, has swiftly come to dominate
the product segments in which it does play a role in
production: enzymes, certain organic acids, and certain
vitamins (see sidebar, " Chemical
processes go live"). In 1994, for example, more than 95
percent of all riboflavin was still chemically synthesized
along traditional lines. Today, 95 percent of it is produced
by genetically enhanced fermentation at a current cost that is
50 percent lower, and with 40 percent less capital expenditure, than conventional techniques
require, in
factories that can turn a profit at one-tenth the scale of
conventional ones.
Both the technical performance of
chemicals produced today from biotechnology and much current
R&D indicate that, by 2010, the price and quality of
biotech-based products will make them competitive in 30
percent of the chemical market as a whole (Exhibit 2). Fine
chemicals will feel the change first and most: by 2010,
biotech-derived fine chemicals will have won a market share of
some 60 percent thanks to their purity, ease of production,
and enhanced efficacy. The next area to be affected will be
some 30 percent of the market for specialty chemicals.
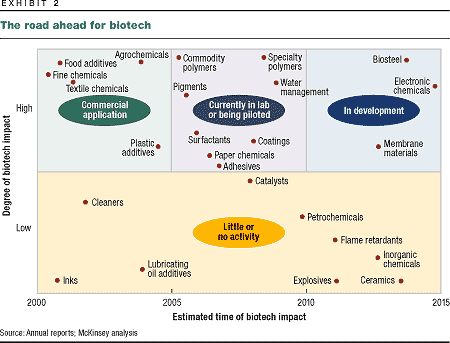
Biotechnology is already used to make
fine chemicals, dyes, food additives, and textile chemicals.
Laboratory and pilot plants have been built to produce
pigments, polymers, and surfactants. But can biotech-based
products really challenge the dominance of polymers and basic
chemicals produced from oil? Both scientific research and
market research suggest that they will, eventually taking over
as much as 50 percent of polymer markets and 15 percent of the
market for basic chemicals.
By 2010 to
2015, the raw-material costs of biopolymers produced from
plants should be no higher than those of petropolymers. It
currently costs around $1.10 per kilogram to produce
polyesters from oil. Production costs of biopolymers made from
corn would be, on average, $0.75 per kilogram, assuming an
extraction rate of 35 percent.4 In the medium term, it will be
fermentation that helps biopolymers enter the commodity
polymer market. Dow Chemical and DuPont plan to start
world-scale production of the biopolymers PLA (polylactic
acid) and PTT (polytrimethylene terephthalate) in 2002 and
2003, at an estimated cost of just under $1 per
kilogram.
Biotechnology-derived products will not be
competing on cost alone. In some areas—certainly in
biopolymers, enzymes, fine chemicals, and new
materials—biotechnology can generate new product features.
 Several of the biopolymers under
development display advantages (biodegradability, for example,
and compatibility with living tissue) that lift them beyond
the category of commodities. Of the more than 100 varieties of
the biopolymer PHA that have been developed, for instance, one
displays a combination of high mechanical resistance and
softness that makes it an ideal substance for fashioning
synthetic human heart valves. Several of the biopolymers under
development display advantages (biodegradability, for example,
and compatibility with living tissue) that lift them beyond
the category of commodities. Of the more than 100 varieties of
the biopolymer PHA that have been developed, for instance, one
displays a combination of high mechanical resistance and
softness that makes it an ideal substance for fashioning
synthetic human heart valves.
Similarly, industrial
applications of newly discovered enzymes that can tolerate
extremely hot, saline, acidic, or alkaline conditions are
forecast to expand the $1.8 billion industrial-enzymes market
by more than 20 percent a year. And the ability of
biotechnology to produce pure chiral molecules will
make it the production technology of choice in fine chemicals,
since it will help allay growing concerns about the safety and
efficacy of pharmaceuticals. Like novel biopolymers, other
revolutionary materials will open new, potentially large
markets. Biosteel for example, a strand of amino acids derived
from spider silk, is stronger than steel but has expansive
properties that make it one of the best materials available
for building earthquake-resistant suspension bridges.
HOW TO DO IT
The business
activities of most chemical companies today are so diverse
that it would be both extremely difficult and unacceptably
risky to apply biotechnology to all of them at once. Before
these companies decide where to focus, they need to assess the
opportunities and threats biotechnology poses to each
business. It will help to answer the following three questions: How much does this business matter to the
company?
What impact will biotechnology have on it? And when will the
impact be felt? Companies can then decide how to respond to
the threats and develop options for exploiting the growth
opportunities.
Those companies that decide to use
biotechnology in production could build capabilities
internally and at the same time acquire small biotech firms
and form alliances. So far, only a few companies have made
much progress in developing and producing biotech-derived
products: Dow Chemical, DSM, and DuPont, among the big
chemical companies, and Genencor International and Novo
Nordisk Pharmaceuticals, among the smaller players. New
entrants still have room to maneuver.
Making a
commitment to biotechnology will profoundly affect the
organization and mind-set of a typical chemical company. Most
biotech businesses devote a much larger part of their
financial and human resources to discovery than chemical
companies do. Chemical conglomerates need to devote greater
resources to generating and developing good ideas if they are
to use biotechnology to grow. This will entail greater
openness to innovation and to risk in the R&D phase,
higher funding for long-term projects, and the building of
links with the external research community to gain access to
new ideas.
Nonetheless, chemical companies, which
already have strong operational skills and customer
relationships, are well positioned to achieve profitable
growth through biotechnology. But they need to start building
biotechnology skills now. As in the pharmaceutical industry,
the first movers have already begun to establish the networks,
the technical leadership, and the intellectual property
reserves that might keep latecomers out.
CHEMICAL PROCESSES GO
LIVE
There are three main types
of biological production processes: fermentation,
bio-catalysis, and plant-based production.
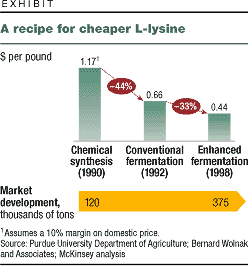
Fermentation uses genetically
engineered microorganisms to produce organic
chemicals. Substituting fermentation for chemical
synthesis can reduce production costs by more than
50 percent. The technique is likely to spread
thanks to improved genetic-engineering methods,
the greater use of multipurpose fermentors (which
let manufacturers scale up production quickly),
and lower costs resulting from the substitution of
light and carbon dioxide for petroleum (exhibit).
Bio-catalysis
means the use of enzymes from living organisms as
catalysts in chemical reactions. Biotechnology is
used to identify and modify appropriate enzymes.
Bio-catalysis reduces the number of steps in the
production process while increasing the purity of
the resulting chemicals. The conventional
production of cefalexin, an antibiotic, takes six
chemical steps, for example. Bio-catalysis
requires just three steps, producing a purer
product at a current cost 60 percent lower than
that of the conventional process and at a capital
cost that is 50 percent lower.
Plant-based
production means using plants themselves as
chemical factories. Genetic engineers are
developing crops better suited to producing
chemicals than their naturally available
counterparts. Using "plants as plants" can lower
manufacturing costs even more than fermentation
does—by more than half, in the long run. Plants,
using light as their source of energy and carbon
dioxide as a raw material, could run any kind of
enzymatic machinery to produce the desired
chemicals. The first ones produced in plants are
already commercially available, but it might be
another 10 to 15 years before they produce
commodity chemicals at competitive costs.
Return
to
above
| |
GLOSSARY
Biotechnology: Techniques
that analyze and manipulate phases in the lives of
living organisms—animals, microorganisms, and
plants—for purposes such as the manufacture of
biological products and improving the
understanding and treatment of disease.
Chiral
molecules: Stereoisomers or molecules
of the same chemical that differ in their
three-dimensional structure.
Commodity chemicals: Single
molecules differentiated only by their level of
purity.
Enzymatic
machinery: Scientists' term for a
number of enzymes used to catalyze a chain of
chemical reactions in a production process.
Enzymes:Proteins that act as
catalysts for the synthesis of specific chemicals
in living organisms.
Life science enterprises:
Businesses that make chemicals used in the fields
of agriculture, animal nutrition and health, and
pharmaceuticals.
Polymers: Plastics and fibers
made of relatively complex molecules
differentiated by their molecular weight and
structure and by their particular properties.
Specialty chemicals: Single
molecules or mixtures valued for their particular
abilities—for example, killing bacteria or slowing
the spread of fire.
| |
Notes
Rolf Bachmann is a principal in McKinsey's
Zurich office; Enrico Bastianelli is a consultant in
the Brussels office; Jens Riese is a consultant in
the Munich office; Wiebke Schlenzka is a consultant
in the Hamburg office. Copyright © 2000 McKinsey &
Company. All rights reserved.
1. That is, companies making basic,
intermediate, and fine-quality commodity chemicals,
polymers, specialty chemicals, and life
science chemicals, plus conglomerates making some
combination of these.
2. Terms printed in italics are defined
in the glossary at the end of this article.
3. This excludes revenue from chemicals
made using traditional fermentation processes unenhanced by
biotechnology—"classic" ethanol, for example.
4. At present, the average extraction
rate in laboratory experiments is only 14 percent, but with
further genetic modifications it could exceed 35 percent.
Some experiments have already extracted biopolymers at
levels exceeding 50 percent.
Copyright © 1992-2002 McKinsey & Company,
Inc.
|
|
| |